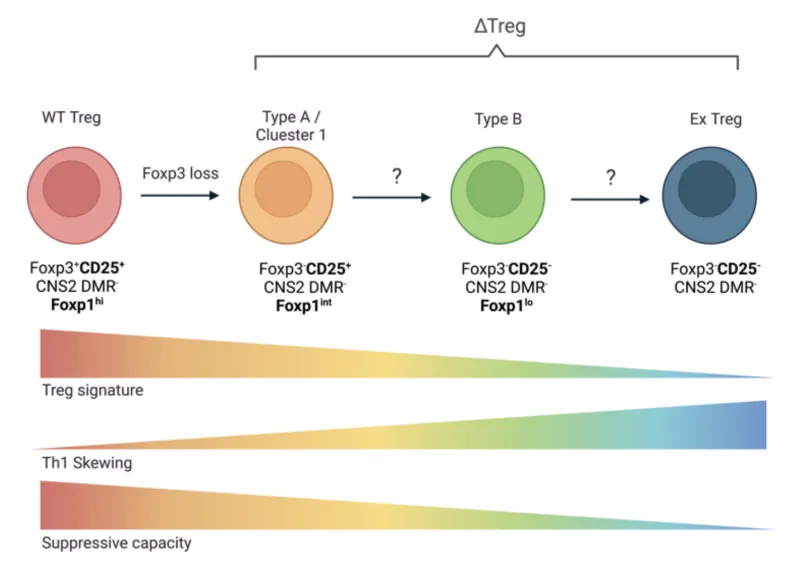
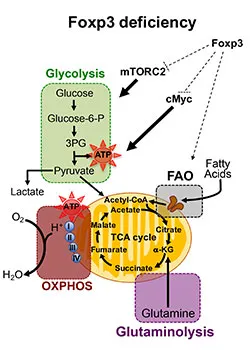
Our central hypothesis is that Foxp3 deficiency destabilizes Foxp3-deficient Treg cells (ΔTreg) towards an effector T (Teff) cell-like program in a stepwise process that can be reversed to re-establish their regulatory functions. Specifically, the degeneration from ΔTreg cells to Teff-like cells involves the transition from a CD25+Foxp1high cell population into the CD25–Foxp1low activated Teff-like cells, both epigenetically related to Treg cells, and ultimately emerge as ex-Treg cells losing the Treg cell epigenetic imprint. Our aim is to understand the molecular mechanisms underlying the different steps of ∆Treg degeneration.