Glycans are the fourth fundamental biopolymer, next to proteins, polynucleotides, and polyalkanes (lipids). In mammals, they are composed of roughly 10 monomeric carbohydrate units, which are strung together and appended to proteins or lipids. Just like RNA, glycans are present in every cell studied to date, across the kingdoms of life. They perform a myriad of essential functions, especially in the context of cell surface events, where complex glycans facilitate the folding and purposeful trafficking of proteins and lipids for secretion or membrane presentation. Thus, processes such as embryogenesis, host-pathogen recognition, and tumor-immune interactions rely on proper glycosylation.
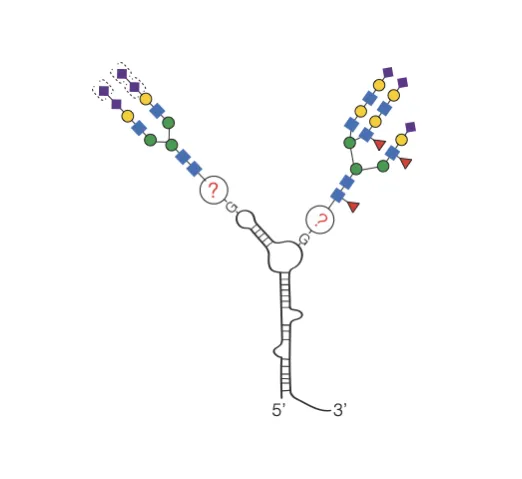
We have recently made the surprising observation that specific RNAs in mammalian cells are modified with glycans (Flynn et al., bioRxiv 2019). Chemical, genetic, and metabolic labeling coupled bioorthogonal chemistry revealed sialylated glycans are conjugated to RNAs in mammalian cells. We term these conjugates glycoRNAs. The glycoRNA conjugate is present in all cell types and tissues tested across human, mouse, and hamster. Sequencing revealed a distinct set of small RNAs including Y RNAs, small nuclear RNAs, and small nucleolar RNAs as modified putatively at guanosine. Chemical, genetic, and enzymatic approaches revelated that sialylated N-glycans, produced by the oligosaccharyltransferase (OST) complex in the ER lumen, modify these RNA transcripts. Overall, these findings point to a common strategy for RNA glycosylation in mammals.